[qwiz qrecord_id=”aglimme-Unit4-1″]
[q]Calculate the molar mass of SO2.
[c]16.00
[c]32.06
[c*]64.06
[c]96.12
[q]Calculate the molar mass of PbCl4
[c]141.8
[c]207.2
[c]242.65
[c*]349.00
[q]Calculate the molar mass of Sn(SO4)2.
[c]214.83
[c]246.71
[c*]310.83
[c]333.54
[q labels=”bottom”]Set up the calculation to find out how many moles of SiCl4 are in 56.5g of SiCl4.
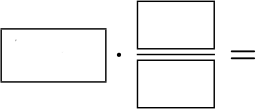
[l]56.5g SiCl4
[fx] The value given in the problem is your starting numbers.
[f*] Great!
[l]1 mol SiCl4
[fx] What you are trying to find goes on the top of the ratio.
[f*] Good!
[l]169.89g SiCl4
[fx] The molar mass goes on the bottom of the ratio.
[f*] Correct!
[q labels=”bottom”]Set up the calculation to find out how many grams of SnI2 are in 0.56moles of SnI2.

[l]0.56mol SnI2
[fx] The value given in the problem is your starting numbers.
[f*] Good!
[l]1mol SnI2
[fx] Since we are starting with moles the 1 mol value goes on the bottom.
[f*] Great!
[l]372.51g SnI2
[fx] What you are trying to find goes on the top of the ratio.
[f*] Great!
[q]How many grams of K2O are in 0.875 moles of K2O?
[c]48.2
[c*]82.4
[c]94.2
[c]108
[q]How many moles of Al(NO3)3 are in 102.6g of Al(NO3)3?
[c*]0.4817
[c]0.5515
[c]1.153
[c]2.076
[q]Calculate the mass percent of nitrogen in 1 mole of N2O5.
[c]20.60
[c*]25.94
[c]46.68
[c]79.40
[/qwiz]